Explanation of Metallizing's Galvanic Protection
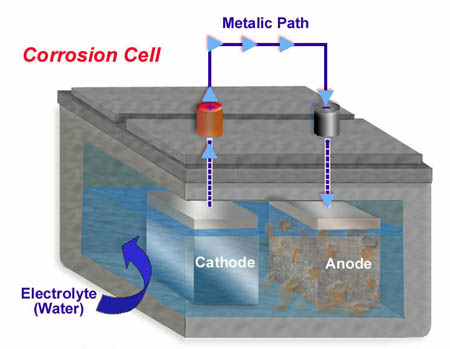
1. The corrosion cell. It works just like a battery works.
A corrosion cell requires four key elements.
- A cathode, or negatively charged particle.
- An anode, or positively charged particle.
- An electrical path.
- An electrolyte, usually water
When all four elements are present, electron flow begins. Electrons flow from the anode through the electrolyte into the cathode, through the electrical path and back to the anode, completing the electrical circuit. As the process continues the anode begins to dissolve. The by-product of this is rust. Rust is the anode disintegrating. The rust becomes liquid as it passesinto the electrolyte. Because half of mild steel is made up of anodic particles, the steel soon falls apart.
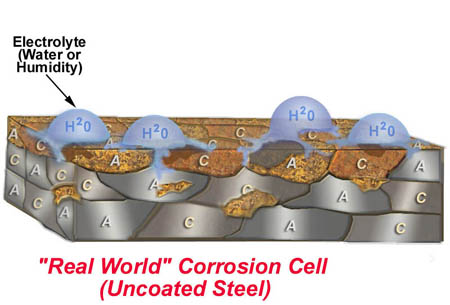
2. Unprotected Steel
This is a real world example of unprotected steel. Mild steel has 3 of the 4 elements needed for a corrosion cell built in. It has anodic and cathodic particles that are touching creating an electrical path. All that is missing is an electrolyte, water. Once water is present, electrons begin flowing from the anode into the electrolyte, then into the cathode, and through the electrical path back to the anode. As this happens the anode begins to dissolve into the electrolyte. The by-product of the anode dissolving is rust.
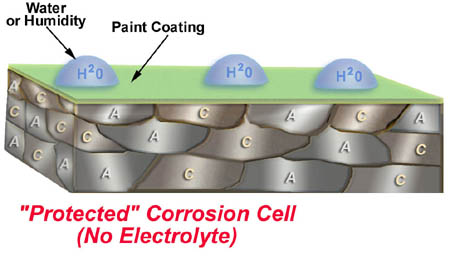
3. Steel protected by paint only. Paint is only a barrier.
Paint protects steel from rusting by forming a barrier on the surface to keep out the electrolyte, water. All of the other 3 elements are still present, anodes, cathodes, and an electrical path. The only reason this steel doesn’t rust is because the paint keeps the electrolyte, water, out. The obvious problem with this method of corrosion prevention is that paint is easily damaged, breaking the barrier.
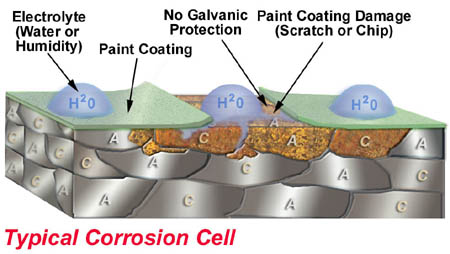
4. Broken paint barrier. All four elements are again present.
At this time rust begins, propagates under the paint causing blisters. Blisters help more of the steel to be exposed to water. Once this cycle has begun it’s only a matter of time before the steel is falling apart. Powder Coating is a beautiful way to paint steel. However, Powder Coating is problematic as a rust preventative because it is still only a barrier coating. Once scratched rust propagates rapidly under the coating.
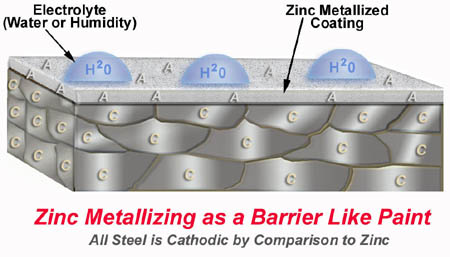
5. Zinc metallizing as a barrier. It's a barrier and much more.
Zinc metallizing, like paint or powdercoating offers a barrier coating that helps to keep water out. This barrier is much stronger than any paint or powdercoating. It is difficult if not impossible to remove this zinc metallized coating. If you take a putty knife and try to scrape the metallizing off, you cannot. You can mar the surface but you cannot get under the zinc and scrape it off. One of the most important concepts of metallizing is the intimate electrical connection between the steel and the zinc. Paint coatings with a lot of zinc in them do not have an intimate connection between the steel and zinc and therefore little or no galvanic protection. Because zinc is such a powerful anode, all of the steel is cathodic by comparison. The steel cannot rust as long as zinc anode is present.
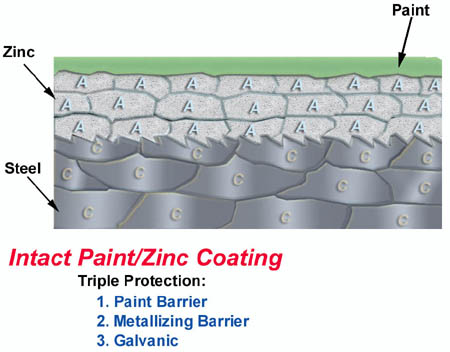
6. Intact zinc metallizing/paint coating. Barrier+Galvanic Protection.
Metallizing provides an extremely strong barrier plus galvanic protection against rust. With paint on top of metallizing, a superior rust preventing coating is established. The metallizing doesn`t have to start working until the paint barrier is broken. A synergism is formed with these two coatings combined. This means that the length of time that the steel will be protected from rusting is greater than the sum of the two protections by themselves. Metallized steel can also be Powder Coated for a beautiful and extremely long lasting finish. Pick a color that you are sure to still like in 15 years!!